Introduction
The NIH estimates that there are about 7000 rare diseases, also known as orphan diseases, and 25- 30 million affected Americans. Of the 7000 identified rare diseases, only about 500 have currently approved treatments. Patients grappling with these conditions often face long and frustrating diagnostic journeys, limited treatment options, and uncertain prognoses. Clinical trials are a key component in developing treatments for these diseases, but they come with their own set of unique challenges. Here we discuss the major challenges associated with rare disease clinical trials and possible ways to overcome or mitigate the issues.
Challenge 1: Recruitment and Retention Difficulties
Recruiting rare disease patients for clinical trials can be time-consuming and costly. Additionally, retaining participants throughout the trial can be challenging due to the wide geographical dispersion of patients and the burden of participation. HealthMappers performed an analysis of factors leading to the premature termination of clinical trials in four rare diseases, revealing that unsuccessful patient recruitment stands out as a primary contributor to trial failure or early termination.
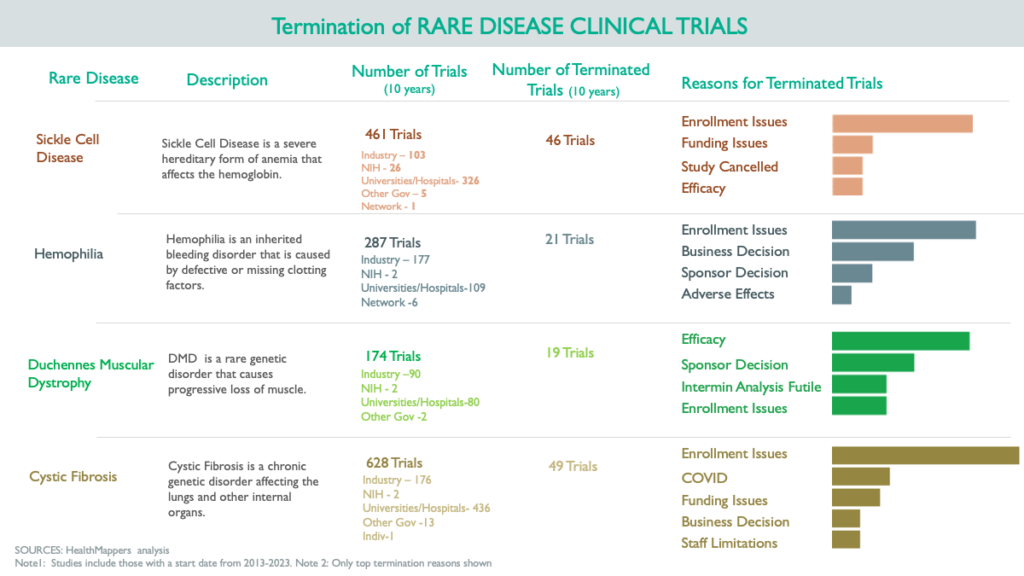
Potential Mitigation Strategies
- Patient Advocacy Groups: Collaboration with patient advocacy organizations raises awareness about clinical trials, improves recruitment efforts, and offers support to reduce patient burden during participation.
- Targeted Recruitment: Finding the right sites to conduct rare disease trials is crucial. For example, In the case of a hemophilia trial, it’s essential to not only consider sites currently engaged in hemophilia trials but also to explore new trial sites. These new trial sites can encompass hematologists with a track record of successfully conducting trials related to other blood disorders, involvement with patient advocacy groups, or established clinical expertise in hemophilia.
- Decentralized Clinical Trials: The recent FDA guidance on decentralized clinical trials is even more essential in the case of rare disease trials. The flexibility offered by decentralized trials could be key in improved recruitment and retention of clinical trial participants by reducing patient burden.
Challenge 2: Limited Patient Populations
One of the most significant hurdles in rare disease clinical trials is the small pool of eligible participants. Traditional trial designs may not be feasible due to the scarcity of patients with the specific disease.
Potential Mitigation Strategies
- Patient Registries: Establishing and maintaining patient registries provides a central database of information about individuals with the rare disease. This can help identify potential trial participants more efficiently.
- Global Collaboration: By partnering with international research institutions and clinics, we can broaden the pool of potential participants and improve the likelihood of identifying suitable candidates. The HealthMappers platform offers a comprehensive view of the clinical trial landscape in your specific disease area. For instance, in the case of a hemophilia trial, it can reveal information about trial sponsors operating in South East Asia—trial site locations, key personnel involved in onsite clinical trial administration, principal investigators contributing to these trials, and potential industry or academic collaborators. Furthermore, the HealthMappers interactive network map provides valuable insights into the connections between these key stakeholders.
Challenge 3: Heterogeneity of Diseases
Rare diseases often exhibit significant heterogeneity, meaning that patients with the same diagnosis may have vastly different symptoms and disease courses. This can make it challenging to define consistent trial endpoints and treatment strategies.
Potential Mitigation Strategies
- Biomarker Development: Biomarker research helps stratify patients based on their disease subtype or genetic profile, allowing for more targeted, precision medicine treatments.
- Adaptive Trial Designs: Adaptive trial designs allow for modifications based on emerging data, enabling adjustments to treatment approaches as needed. Recent guidance has suggested single arm trials without a placebo arm.
Challenge 4: Regulatory Hurdles
Navigating the regulatory landscape for rare disease therapies can be complex. Regulatory agencies need to strike a balance between ensuring safety and expediting the approval of life-saving treatments.
Potential Mitigation Strategies
- Early Engagement: Engaging with regulatory agencies early in the drug development process to clarify expectations, discuss trial design, and address potential issues proactively.
- Accelerated Approval Pathways: Accelerated approval pathways for promising rare disease therapies, allows for faster access to treatments for patients in need.
Clinical trials for rare diseases, though fraught with challenges, are essential for advancing our understanding and treatment options for these often-neglected conditions. At HealthMappers we are passionate about bringing treatments for rare diseases to the patients that need them.
We provide comprehensive solutions exploring the rare disease space, including the latest publications, clinical trials, industry partners, disease experts, advocacy groups, conferences, networks of people and institutions working in this space, and potential clinical trial sites for your study. Contact us to learn more about how we can help!


