INTRODUCTION
Based on recent data on clinical diversity, plus insights from both the FDA and WHO, global clinical trials collecting rigorous data are essential for understanding and dispersing advances in healthcare with equity.1-2.7 Using Idiopathic Pulmonary Fibrosis (IPF) as a case study, HealthMappers analyzed the research landscape to discover the real-world diversity of the global research landscape.
WHY IPF?
First, it meets the criteria of a rare disease where diverse patient recruitment is essential. Second, it has the highest prevalence in South Korea—a non-North America, non-European country. Finally, IPF is also experiencing increased awareness globally.
ABOUT IPF PREVALENCE
IPF is a rare, progressive lung disease with no cure but with quality of life treatments available. Globally, the prevalence is 13 to 20 cases per 100,000 people. This includes 30,000 to 40,000 new cases expected to be diagnosed each year.3
Even more alarming is the fact that prevalence is on the rise, possibly due to the increased awareness and other unknown reasons.4-5 Notably, South Korea has the world’s highest prevalence. One aggregate study found that South Korea no longer even met rare disease status. In the US, prevalence among veterans increased 160% from 2010 to 2019.5 One group showed the clear gaps in knowledge regarding IPF prevalence for the regions of Africa, South Asia, South America and the Middle East. Data simply was not available for the incidence or predicted prevalence in these regions.5-7
CASE STUDY
HealthMappers analyzed the “knowledge gap” regions, along with South Korea, in the research landscape. Regions included were the Middle East, Africa, Asia (minus China and Japan), Southeast Asia, Eastern Europe, Mexico and South America. We chose the measure of scientific share of voice to reflect not just participation, but potential strategic / decision-making involvement in IPF research. Our results were surprising.
- 13% scientific share of voice (SSoV) in clinical trial publications
- 16% SSoV in research articles and guidelines activities.
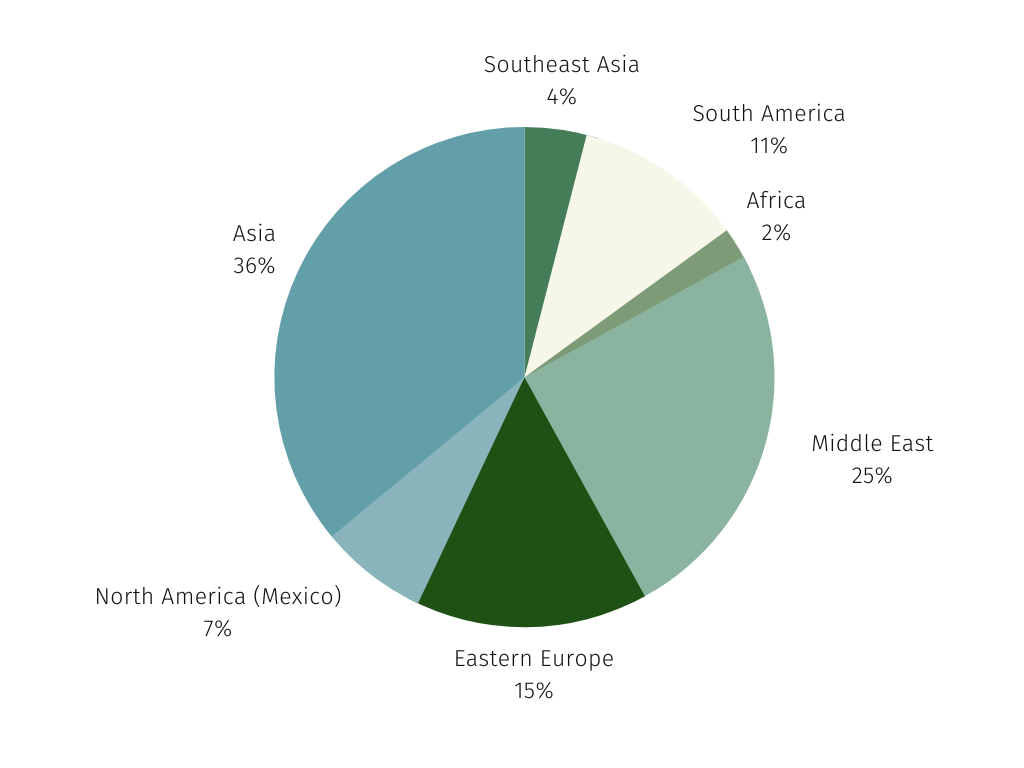
Considering this SSoV includes South Korea, the country with the highest prevalence, the numbers are noticeably small in these regions. However, with new reviews and analysis of the research coming from these regions we can choose to be encouraged. They show a start to global diversity in research activities and provide hope that diverse research, both in clinical trials and real world, is starting to fill that regional knowledge gap. Insights such as these are crucial, not just in the rare disease population, but for creating truly diverse patient recruitment.
References
- International Clinical Trials Registry Platform (ICTRP) (who.int)
- Clinical Trial Diversity | FDA
- IPF: Statistics, Facts, and You (healthline.com)
- Global Idiopathic Pulmonary Fibrosis Market $10.1 Billion by 2029 (ihealthcareanalyst.com)
- What Do We Need to Know About Rising Rates of Idiopathic Pulmonary Fibrosis? A Narrative Review and Update – PMC (nih.gov)
- Global incidence and prevalence of idiopathic pulmonary fibrosis – PMC (nih.gov)
- Underrepresentation Overconsumption Exploring The Gap In Global Clinical Trial Diversity (clinicalleader.com)


